How Synthetic Oil Is Made
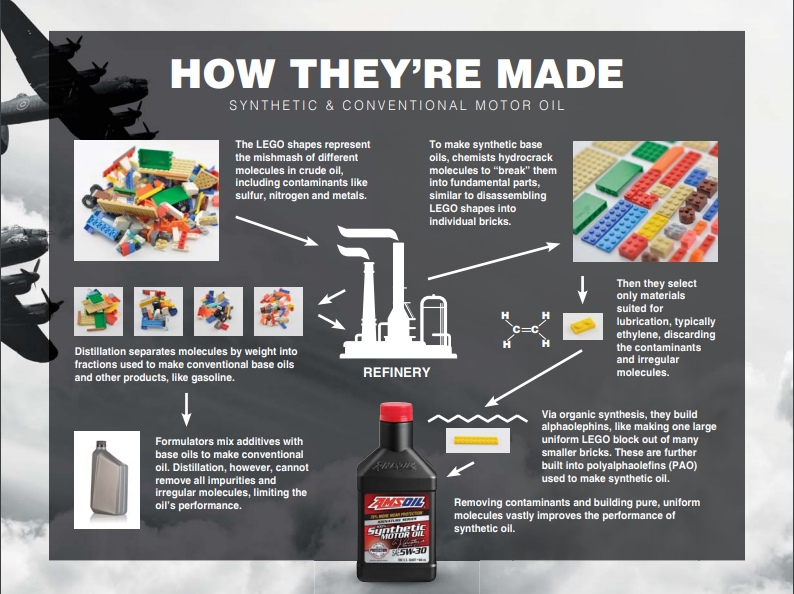
Building Blocks
For either Conventional oil or Synthetic oil, start with Crude Oil. Think of crude oil like a pile of Lego™ blocks haphazardly connected to form various shapes of different sizes (see picture). Each differently shape and colored block represents different molecules including carbon, sulfur, nitrogen, and oxygen.
Conventional Oil
is made by distilling crude oil. The distillation process is shown on the left side of the picture. Distillation separates the molecules by size with a pile for each size of block. Each pile has various molecules of similar sizes. There are a lot of impurities in each pile, one of which is used as the base stock for conventional motor oil. The manufacturer then adds additives to produce the finished product.
Synthetic Oil
is “built”, not distilled. The chemical "building" process is show on the right side of the picture. This means formulators start by using a chemical process to “crack” the crude oil into individual molecules as shown on the right side of the picture. They select only pure uniform molecules, typically ethylene if formulating a polyalphaolefin (PAO) based oil. Using organic synthesis, chemists use ethylene to build larger molecules called alphaoleins which are used to build the PAO base stock. Like conventional oil, the manufacturer then adds additives to produce the finished product.